

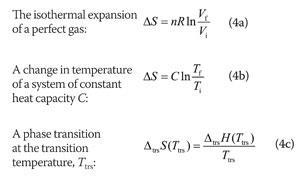
As long as the same amount of thermal energy was gained by the frying pan and lost by the water, the first law of thermodynamics would be satisfied. Suppose that a hot frying pan in a sink of cold water were to become hotter while the water became cooler. Now consider the same process in reverse. This transfer of heat from a hot object to a cooler one obeys the first law of thermodynamics: energy is conserved. Eventually both objects will reach the same temperature, at a value between the initial temperatures of the two objects. If a hot frying pan that has just been removed from the stove is allowed to come into contact with a cooler object, such as cold water in a sink, heat will flow from the hotter object to the cooler one, in this case usually releasing steam. Let’s consider a familiar example of spontaneous change. This information, however, does not tell us whether a particular process or reaction will occur spontaneously. You also learned previously that the enthalpy change for a chemical reaction can be calculated using tabulated values of enthalpies of formation. Changes in the internal energy (ΔU) are closely related to changes in the enthalpy (ΔH), which is a measure of the heat flow between a system and its surroundings at constant pressure.
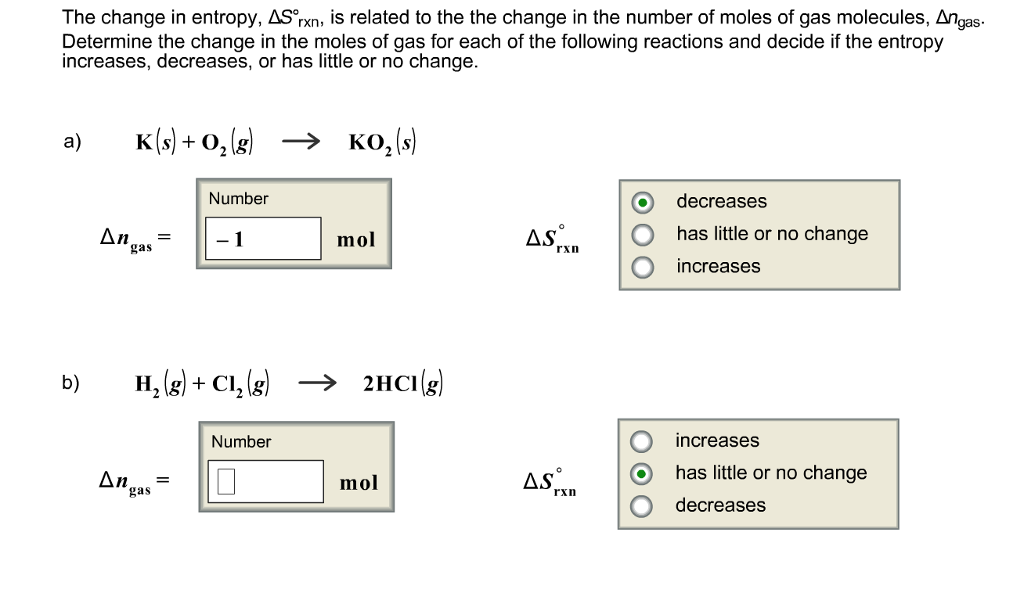
The first law of thermodynamics governs changes in the state function we have called internal energy (\(U\)). To understand the relationship between internal energy and entropy.


 0 kommentar(er)
0 kommentar(er)
